Abstract
Background: The number of older adults (age ≥65) is growing rapidly worldwide and these patients are undergoing hematopoietic cell transplant (HCT) at historically unprecedented rates. The advent of post-transplant cyclophosphamide-based haploidentical related donor (HRD) protocols has expanded HCT access for patients without an HLA-matched related donor (MRD) or unrelated donor (MUD). However, comparative, diagnostically diverse studies in older adults are lacking.
Methods: We retrospectively identified all patients age ≥65 undergoing MRD, MUD or HRD peripheral blood stem cell HCT at our institution between October 2012 and June 2017 using an institutional database. Data were gathered via manual chart review. Pre-transplant comorbidity was prospectively assessed using the HCT-Comorbidity Index (HCT-CI) and Karnofsky Performance Score (KPS). Disease risk was retrospectively scored using the disease risk index (DRI). Acute graft-versus-host disease (aGvHD) was retrospectively graded per accepted criteria.
Categorical variables were compared using the χ2 test or, in case of sparse cells, Fisher's exact test. Continuous variables were analyzed using the Kruskal-Wallis H test. A pre-specified set of variables were screened for inclusion in a multivariate Cox Proportional Hazards (CPH) model of survival, with p < 0.10 used as the cutoff for model entry (table 2). Cumulative incidence (CI) of aGvHD, relapse and treatment-related mortality (TRM) were calculated using the method of Fine and Grey. Patients with active disease at transplant were excluded from analysis of TRM and relapse if they had morphologic or cytogenetic evidence of disease on day +30 bone marrows or expired before undergoing testing. CI analysis was performed using R (v. 3.4.1). All other analyses were performed in SPSS (v. 24).
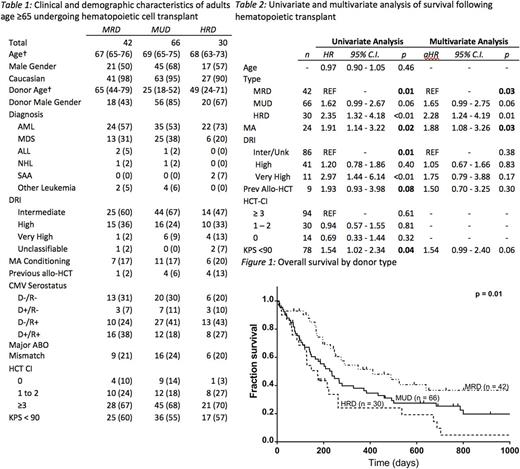
Patients: We identified 42, 66 and 30 patients undergoing MRD, MUD and HRD HCT. Median follow-up in all patients was 8.2 months (range: 0.1 - 52.6), 6.1 months (0.2 - 49.7) and 4.5 months (0.3 - 55.9), respectively, and 15.1 months (2.3 - 52.6), 21.7 months (1.3 - 49.7) and 6.1 months (1.1 - 55.9) in patients surviving to last follow-up. Clinically, the cohorts were well-balanced, with no significant difference in diagnosis, conditioning intensity, disease risk or comorbidity (table 1). MRD patients were younger than MUD and HRD patients (p = 0.01). Donors for MUD HCT were younger (p <0.001) and more likely to be male (p < 0.001).
Results: At last follow-up, 47 patients (34%) were alive. The unadjusted 1-year overall survival (OS) was 51% (95% C.I. 35% - 67%), 38% (95% C.I. 26% - 50%) and 24% (95% C.I. 6% - 42%) in the MRD, MUD and HRD cohort, respectively. On Kaplan-Meier analysis, OS was significantly different between groups (Figure 1, log-rank: p = 0.01). On pairwise analysis, MRD was significantly superior to both HRD HCT (p < 0.01) and MUD HCT (p = 0.05). On univariate analysis, donor type, DRI, conditioning intensity and KPS < 90 were associated with survival (table 2). Previous allogeneic HCT (p = 0.08) was also included in the CPH model. On multivariate analysis, only donor type and conditioning intensity remained significant predictors of survival. Myeloablative (MA) conditioning was associated with significantly inferior survival (aHR: 1.8, 95% C.I. 1.08 - 3.26). Compared to MRD, HRD had significantly higher mortality (aHR: 2.28, 95% C.I. 1.24 - 4.19). MUD HCT had a trend towards inferior survival which did not reach statistical significance (aHR: 1.65, 95% C.I. 0.99 - 2.75).
Eighty-three percent, 86% and 93% of MRD, MUD and HRD patients were included in relapse and TRM analysis. The CI of relapse at 1 year was 38%, 25% and 44% respectively (p = 0.40). The CI of NRM at 1 year was 16%, 44% and 34% (p = 0.07). Grade II-IV aGvHD at 100 days was significantly higher in the MUD cohort (38%) versus the MRD (10%) and HRD (10%) cohorts (p = 0.01). Similarly, grade III-IV aGvHD was significantly higher among MUDs (25% vs. 7% vs. 3%, p = 0.03).
Conclusion: Overall, our analysis supports extending the paradigm of HLA-matched sibling donors as the gold standard in allogeneic HCT to adults age ≥65. It also raises questions about the role of myeloablative conditioning in older adults. Our analysis is limited by sample size and relatively short follow-up, especially among the HRD cohort. Both registry-based and prospective studies are needed to further define risk factors for poor outcomes among older adults and identify possible interventions.
Vij: Celgene: Honoraria; Takeda: Honoraria, Research Funding; Jazz: Honoraria; Abbvie: Honoraria; Bristol-Meyers-Squibb: Honoraria; Janssen: Honoraria; Konypharma: Honoraria; Amgen: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal